Concept of PERISCOPE
The development and licensing of the next generation of improved vaccines depends on 1) a more thorough understanding of the immune response to infection and vaccination 2) identification of biomarkers of protective immunity and 3) development of human and pre-clinical models as well as bioassays to predict and evaluate vaccine efficacy.
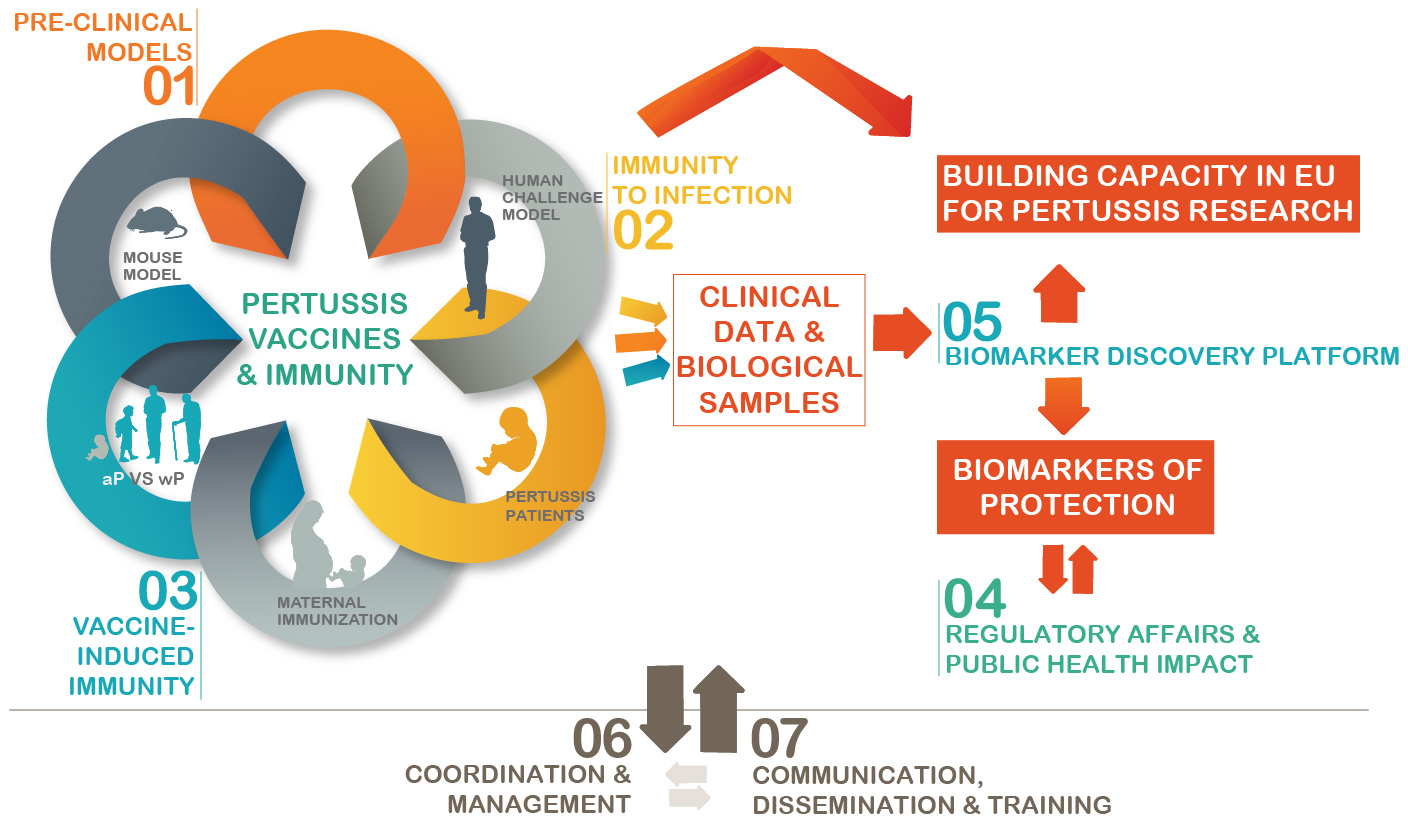
PERISCOPE will develop novel functional antibody and cellular assays and employ cutting edge science to characterize innate and adaptive immune responses. Immune responses will be characterized through clinical trials that will compare aP with wP vaccine in a range of age groups, including infants. The impact of immunization in pregnancy on subsequent immune responses of infants to aP or wP will be determined in a European and an African setting. Experimental human and pre-clinical B. pertussis challenge models will be developed to identify immune signatures that predict protection against B. pertussis colonisation and/or disease. These signatures will then be compared to immune responses following vaccination. Potential biomarkers of protection against colonisation and/or disease will subsequently be evaluated in the human challenge model, cohorts of naturally infected individuals, including exposed household contacts, and in the pre-clinical model. A second pre-clinical model will be used to gain insight into the underlying mechanisms of protection.